Title
Characterisation of emulsion formulations
Objective
To determine:
1. The effects of HLB surfactant on the stability of
the emulsion.
2. The effects of different oil phases used in the
formulation on the physical characteristics and stability of the emulsion.
Introduction
An emulsion is a thermodynamically unstable two-phase system consisting
of at least two immiscible liquids. It contains at least two immiscible liquid
where one of them is dispersed and dispersion medium. The dispersed liquid is known
as the internal or discontinuous phase, whereas the dispersion medium is known
as the external or continuous phase. In general, emulsion can be categorized
into 2 types, oil in water emulsion (o/w) and water-in-oil emulsion (w/o). . An
o/w emulsion is generally formed if the aqueous phase constitutes > 45% of
the total weight, and a hydrophilic emulsifier is used. Conversely, where water
or aqueous solutions are dispersed in an oleaginous medium, the system is known
as a water-in-oil (w/o) emulsion. W/O emulsions are generally formed if the
aqueous phase constitutes < 45% of the total weight and an lipophilic
emulsifier is used.
The HLB method (hydrophilic-lipophilic balance) is
used to determine the quantity and type of surfactant that is needed to prepare
a stable emulsion. Every surfactant is given a number in the HLB scale, that is
form 1 (lipophilic) to 20 (hydrophilic). Usually a combination of 2 emulsifying
agent is used to form a more stable emulsion. HLB value for a combination of
emulsifying agents can be determined by using the following formula.
The consistency of
emulsions varies from easily pourable liquids to semisolid creams. Thus, emulsions are used in many
routes of administration. Most commonly, emulsions are used for topical
administration. Emulsions are also used as an ointment bases and intravenously
administered as part of parenteral nutrition therapy.
Apparatus
and materials
a. Apparatus
8 test tubes, a 50ml measuring cylinder, 2 sets of
pasture pipettes and droppers, vortex mixer, weighing boat, 1 set of mortar and
pestle, light microscope, microscope slides, 1 set of 5ml pipette and bulb, 1
50ml beaker, a 15ml centrifugation tube, centrifugation apparatus, viscometer,
water bath (45 celsius) and refrigerator (4 celsius).
b. Materials
Palm oil, Arachis oil, Olive oil, Mineral oil,
Distilled water, Span 20, Tween 80, and Sudan III solution (0.5%)
Procedures:
1. Each test tube was labelled and marked 1 cm from
the base of the test tube.
2. 4ml of oil was mixed and 4ml of distilled water
into the test tube.
Group
|
Oil
|
1,5
|
Palm
oil
|
2,6
|
Arachis
oil
|
3,7
|
Olive
oil
|
4,8
|
Mineral
oil
|
3. Span 20 and Tween 80 were added into the mixture
of oil and water (refer Table 2). The test tube was closed and its content was
mixed with vortex mixer for 45 seconds. The time needed for the interface to
reach 1cm was recorded. The HLB value for each sample was recorded. Steps 1-3
were recorded in order to obtain an average HLB value of a duplicator.
Tube
no
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
Span
20
(drops)
|
15
|
12
|
12
|
6
|
6
|
3
|
0
|
0
|
Tween
80 (drops)
|
3
|
6
|
9
|
9
|
15
|
18
|
15
|
0
|
Table
2
4. A few drops of Sudan III solution were added to
(1g) emulsion formed in a weighing boat and mixed homogenously. The spread of
the colour in the sample was compared. Some of the sample was spreaded on a
microscope slide and observed under light microscope. The appearance and
globule size formed was described and drawn.
5. Mineral Oil Emulsion (50 ml) was prepared from
the formulation below by using wet gum method according to Table 3a and 3b.
Mineral
Oil
|
(refer
Table 3b)
|
Acacia
|
6.25g
|
Syrup
|
5ml
|
Alcohol
|
3ml
|
Distilled
water qs
|
50ml
|
Table
3a
Emulsion
|
Group
|
Mineral
Oil (ml)
|
I
|
1,5
|
20
|
II
|
2,6
|
25
|
III
|
3,7
|
30
|
IV
|
4,8
|
35
|
Table
3b
6. 40g of emulsion was placed intoa 50ml beaker and
homogenized for 2 minutes using a vortex mixer.
7. 2g of emulsion (before and after homogenization)
was taken and placed into a weighing boat and labelled. A few drops of Sudan
III solution was added and mixed. The texture,
consistency, degree of oily appearance and the spreading of colour in the sample
under the light microscope was stated and compared.
8. The viscosity of the emulsion formed after
homogenization (15g in 50ml) was determined using viscometer that is calibrated
with “Spindle” type LV-4. The sample was exposed to 45 celsius (water bath) for
15 minutes and then to 4 celsius (refrigerator) for another 15 minutes. After
the exposure to the temperature cycle is finished and the emulsion had reached
room temperature (10-15 minutes), the viscosity of emulsion is determined. Step
8 was repeated again and an average value was obtained.
Readings
|
Viscosity(cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before
Temperature
Cycle
|
|||||||
After
Temperature Cycle
|
|||||||
Difference
(%)
|
|||||||
Table
4
9. 5g of homogenized emulsion was placed into a centrifugation
tube was placed and centrifuged (4500 rpm, 10 minutes, 25 celsius). The height
of the separation formed was measured and the ratio of the height separation
was determined.
Mineral
Oil (ml)
|
Ratio of separation
|
Average
|
Ratio
of separation phases
|
|||
20
|
||||||
25
|
||||||
30
|
||||||
35
|
||||||
Result
For
Palm Oil:
|
Palm oil
|
||||||||
|
Tube
number
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
|
Span
20 (drops)
|
15
|
12
|
12
|
6
|
6
|
3
|
0
|
0
|
|
Tween
80 (drops)
|
3
|
6
|
9
|
9
|
15
|
18
|
15
|
0
|
|
HLB
value
|
9.67
|
10.73
|
11.34
|
12.44
|
13.17
|
14.09
|
15.00
|
0.00
|
|
Time
of phase separation (min)
|
---
|
---
|
86
|
60
|
80
|
113
|
85
|
15
|
“---” = Time taken greater than 130
minutes (To find the average, 130
minutes used to represent the time taken for interphase to reach 1cm for “---”
For Olive Oil:
|
Olive oil
|
||||||||
|
Tube number
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
|
Span 20 (drops)
|
15
|
12
|
12
|
6
|
6
|
3
|
0
|
0
|
|
Tween 80 (drops)
|
3
|
6
|
9
|
9
|
15
|
18
|
15
|
0
|
|
HLB value
|
9.67
|
10.73
|
11.34
|
12.44
|
13.17
|
14.09
|
15.00
|
0.00
|
|
Time of phase separation (min)
|
---
|
---
|
57
|
11
|
32
|
14
|
37
|
0
|
“---” = Time taken greater than 130
minutes (To find the average, 130
minutes used to represent the time taken for interphase to reach 1cm for “---”
For Mineral Oil:
|
Mineral oil
|
||||||||
|
Tube number
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
|
Span 20 (drops)
|
15
|
12
|
12
|
6
|
6
|
3
|
0
|
0
|
|
Tween 80 (drops)
|
3
|
6
|
9
|
9
|
15
|
18
|
15
|
0
|
|
HLB value
|
9.67
|
10.73
|
11.34
|
12.44
|
13.17
|
14.09
|
15.00
|
0.00
|
|
Time of phase separation (min)
|
---
|
---
|
69
|
61
|
56
|
46
|
0
|
0
|
“---” = Time taken greater than 130 minutes (To find the average, 130 minutes
used to represent the time taken for
interphase to reach 1cm for “---”
For arachis oil (Group 2 B):
Group 2( Arachis
oil)
|
||||||||
Tube number
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
Span 20 (drops)
|
15
|
12
|
12
|
6
|
6
|
3
|
0
|
0
|
Tween 80 (drops)
|
3
|
6
|
9
|
9
|
15
|
18
|
15
|
0
|
HLB value
|
9.67
|
10.73
|
11.34
|
12.44
|
13.17
|
14.09
|
15.00
|
0.00
|
Time of phase separation (min)
|
---
|
79
|
75
|
35
|
27
|
40
|
20
|
0
|
HLB value =
|
[(quantity of surfactant 1)(HLB of surfactant 1) +
(quantity of surfactant 2)
(HLB of surfactant 2)]
|
quantity of
surfactant 1 + quantity of
surfactant 2
|
Calculation of HLB
values:
HLB value for Span 20
= 8.6
HLB value for Tween 80
= 15.0
Therefore, example of calculation:
HLB value for Tube 1 = (15 x 8.6) + (3 x 15.0)
(15 + 3)
=
9.67
HLB value for Tube 2 = (12 x 8.6) + (6 x 15.0)
(12 + 6)
=
10.73
HLB value for Tube 3 = (12 x 8.6) + (9 x 15.0)
(12 + 9)
= 11.34
HLB value for Tube 4 = (6 x 8.6) + (9 x 15.0)
(6 + 9)
=
12.44
HLB value for Tube 5 = (6 x 8.6) + (15 x 15.0)
(6 + 15)
=
13.17
HLB value for Tube 6 = (3 x 8.6) + (18 x 15.0)
(3 + 18)
=
14.09
HLB value for Tube 7 = (0 x 8.6) + (15 x 15.0)
(0 + 15)
=
15.00
HLB value for Tube 8 = (0 x 8.6) + (0 x 15.0)
(0 + 0)
=
0.00
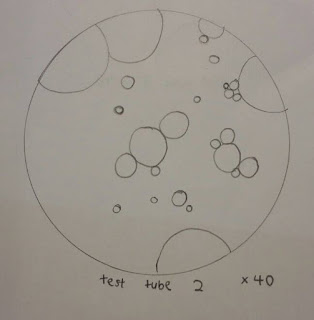
For arachis oil:
Appearance and globule size under light microscope
Test tube
|
Shape
|
Globule size
|
Spread of colour
|
1
|
Round globule
|
Small
|
Not Even
|
2
|
Round globule
|
Big
|
Even
|
3
|
Round globule
|
Tiny
|
Even
|
4
|
Round globule
|
Small
|
Not Even
|
5
|
Round globule
|
Tiny
|
Even
|
6
|
Round globule
|
Small
|
Even
|
7
|
Round globule
|
Tiny
|
Not Even
|
8
|
Round globule
|
Tiny
|
Not Even
|
2g of emulsion before and after homogenization
For
20ml mineral oil
|
Before
homogenization
|
After
homogenization
|
Texture
|
Non
homogenous (spacious)
|
Homogenous
(packed)
|
Consistency
|
Less
consistent-crystal clump together
|
More
consistent-crystals dispersed
|
Degree
oily appearance
|
More
greasy
|
Less
greasy
|
Spreading
of colour
|
Less spreading
|
More
spreading
|
For
25 ml mineral oil
|
Before
homogenization
|
After
homogenization
|
Texture
|
Non
homogenous
|
Clear
and homogenous
|
Consistency
|
Less
consistent
|
More
consistent
|
Degree
oily appearance
|
More
greasy
|
Less
greasy
|
Spreading
of colour
|
Less
even distribution
|
Better
even distribution
|
For
30ml mineral oil
|
Before
homogenization
|
After
homogenization
|
Texture
|
Course
and not homogenous
|
Smooth
and homogenous
|
Consistency
|
Not
consistent,less viscous
|
Consistent
|
Degree
oily appearance
|
More
greasy,spherical globules
|
Les
greasy and aspherical globules
|
Spreading
of colour
|
Unevenly
spreading
|
Evenly
spreading
|
For
35ml mineral oil
|
Before
homogenization
|
After
homogenization
|
Texture
|
More
watery and non viscous
|
Less
watery and more viscous
|
Consistency
|
Not
consistent and non viscous
|
Consistent
and mor viscous
|
Degree
oily appearance
|
More
greasy and more globules
|
Less
greasy and globules
|
Spreading
of colour
|
Unevenly
distribution
|
Evenly
distribution
|
For Viscosity Of Emulsion:
20 ml of mineral oil
Readings
|
Viscosity
(cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before Temperature Cycle
|
42
|
42
|
36
|
24
|
30
|
24
|
33
|
After Temperature Cycle
|
48
|
45
|
40
|
30
|
38
|
36
|
39.5
|
Difference(%)
|
19.7
|
||||||
25ml of mineral oil
Readings
|
Viscosity
(cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before Temperature Cycle
|
30
|
30
|
30
|
30
|
30
|
30
|
30
|
After Temperature Cycle
|
44
|
46
|
46
|
48
|
45
|
46
|
45.8
|
Difference(%)
|
52.7
|
||||||
30ml of mineral oil
Readings
|
Viscosity
(cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before Temperature Cycle
|
60
|
48
|
54
|
48
|
48
|
54
|
52
|
After Temperature Cycle
|
83
|
70.4
|
80.1
|
72.4
|
70.4
|
80.1
|
76.1
|
Difference(%)
|
46.3
|
||||||
35ml of mineral oil
Readings
|
Viscosity
(cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before Temperature Cycle
|
3.6
|
3.9
|
6.9
|
3.9
|
18.3
|
17.1
|
9.0
|
After Temperature Cycle
|
14.4
|
19.2
|
10.8
|
8.4
|
7.2
|
8.4
|
11.4
|
Difference(%)
|
26.7
| ||||||
Mineral Oil
(ml)
|
Ratio of
separation phase
|
Average
|
Ratio of
separation phase
|
||
20
|
4.00
|
3.50
|
4.00
|
3.83
|
0.77
|
25
|
3.00
|
3.30
|
3.50
|
3.27
|
0.66
|
30
|
2.86
|
2.70
|
2.86
|
2.81
|
0.56
|
35
|
2.25
|
2.25
|
2.15
|
2.22
|
0.44
|
Stable emulsion means longer time for phase separation to
occur. For palm oil, the HLB value
that brings to stable emulsion is 11.34. This means that the stable emulsion of
palm oil can be prepared by adding 12 drops of Span 20 and 9 drops of Tween 80.
For arachis oil, the HLB value that
can give stable emulsion is 11.34. For olive
oil, the HLB value that can give stable emulsion is 11.34. For mineral oil, the HLB value that can
give stable emulsion is 10.70. These show that different oils with different
HLB values require different combination of surfactants in order to produce a stable
emulsion.
From
the experiment for arachis oil which is done by our group, it was found that
emulsion from test tube 1, 2 and 3 where the HLB values are 9.67, 10.73 and
11.34 respectively give the most stable emulsions. Their phase separation time
is very long if compared to the other test tubes. Thus, the corresponding HLB
value of 9-13 gives the most effective surfactant for the oils used in this
experiment. Appropriate HLB value is important in determining the stability of
the emulsion. Meanwhile, emulsions from tube 7 and 8 give the lowest stability
where the phase separation time is the shortest. This is because the absence of
surfactant as an emulsifying agent in tube 7 and 8. Surfactant enhances the
distribution of oily phase into the aqueous phase (o/w emulsion) or the
distribution of aqueous phase into the oily phase (w/o emulsion).
A
low HLB value have more hydrophobic group while the high HLB value have more
hydrophilic group. Span is hydrophobic and is used to make w/o emulsion.
Meanwhile, tween is hydrophilic and is used to form o/w emulsion. In the
stabilization of oil globules, it is essential that there is a degree of
hydrophilicity to confer an enthalpic stabilizing force and a degree of
hydrophobicity to secure adsorption at the interface. So, a combination of both
offers a suitable HLB value which matches with the system and produces a stable
emulsion. The HLB value of the surfactant that fulfills the required HLB value
of the emulsion will form the stable emulsion and vice versa. The emulsion is
formed by reducing the surface tension of the two immiscible liquid through the
presence of surfactant at the interface. Generally, a combination of surfactant
is better than a single surfactant, which produces more stable emulsion.
During
this experiment , we did mixing the emulsion homogenously.Before
homogenization, the globules are not in uniform size and are coarse. There has
a combination of small, intermediate and large size globules. However, the size
of globules becomes uniform after homogenization and all globules are in
smaller size. About the greasiness, the emulsion is greasy and less viscous
before homogenization due to the reason of unemulsified oil. However, the
emulsion becomes smoother and more viscous after homogenization. This is
because the sample tube is spun in the high rate and breaks the globules into
smaller sizes. Besides that, the emulsion is less consistent before
homogenization. However, the consistency of the emulsion is increased and the
degree of oily are decreased after homogenization because during
homogenization, forces are applied to the emulsion and thus it causes a better
emulsifying effect to take place. After adding Sudan test III solution, the
colour of the emulsions becomes milky. It shows good colour dispersion in the
emulsions.
Sudan
test is a group of azo compound used as biological stains for fat. It is used
to show the shape and physical characteristic of oily emulsion. It can
differentiate which emulsion is oil-in-water emulsion or water-in-oil emulsion
by determining the amount of globules in red colour and the colourless
globules. Sudan solution is a red colour solution. It is dissolved in oily
phase of the emulsion. So, it will cause the oily globules stain in red colour.
The colour dispersion of the emulsions before homogenization is not consistent.
However after the homogenization, the colour of dispersion is more consistent.
Thus, the emulsion formed is considered as o/w emulsion.
For viscosity of emulsion after
homogenization
Graph 1.
Based on the graph 1, the percentage difference of viscosity (%) increases as the amount of mineral oil used increases. But percentage difference of viscosity (%) started to decrease when using 30 and 35 ml of mineral oil. All type of emulsion is exposed to the same temperature and they have the same volume, but different proportion of emulsifying agent, oil and water proportions. The percentage difference is the difference between the viscosity before and after the temperature cycle. For 20 mL of mineral oil, the percentage difference of viscosity is small which is 19.70% because the emulsion is not too viscous. For 25 mL of mineral oil, the percentage difference of viscosity is 52.67% which is much higher than mineral oil of 20 mL and that is because the emulsion is getting more viscous when adding more mineral oil into it. For 30 and 35 mL of mineral oil used, the percentage difference is 46.30% and 26.70% which is lower than 25 mL of mineral oil used. So this might be due to some errors occurs during handling the experiment because the percentage difference should be higher than 25 mL of mineral oil used. The percentage difference of viscosity (%) should increase with increasing amount of mineral oil used.
Viscosity
means a quantity expressing the magnitude of internal friction , as measured by
the force per unit area resisting a flow in which parallel layers unit distance
apart have unit speed relative to one another . In simple term , it can be
explain as a measurement of a fluid’s internal resistance to flow.
Besides,
in this experiment, the other objective is to test the physical effects and
stability on the emulsion formulation due to different amount of emulsifying
agent. Thus it will be tested at different temperature , and can also effect the
emulsifying agent. Emulsifying agent will prevent coalescence of droplets and
maintain the individual droplets in the continuous phase. It also acts by
adsorb onto the oil-in-water interface and lower the surface interfacial
tension. In addition, emulsifying agent tends to promote dispersion of the
phase in which they do not dissolve very well.
Increase
in amount of mineral oil used in an emulsion will increase amount of acacia
that will be use as an emulsifying agent . This is due to the ratio 4:2:1. The
4 part represent oil part , 2 part for the water while 1 part is for the
emulsifying agent.
Since
acacia does not dissolves in oil, so this will form oil-in-water emulsion. Increase
in temperature will also increase droplet size and reduce viscosity. The
increasing of droplet size will make the emulsion difficult to disperse and
easily separate to two layers . While in cold temperature , the emulsion will
become thicker and viscous and difficult to be separate in two layer.
The
value of emulsion after the temperature cycle is in greater value than before
the temperature cycle. This happen due to loss of some proportion of the volume
during heating process. During heating process the molecule will vibrate but
interact less. Thus the viscosity value is low.
For the ratio of separation phase
Centrifugation
of emulsion is used to separate the oil phase from the aqueous phase. From the
result obtained , it proved that the phase of separation ratio will be lower if
the more volume of mineral used in the formulation. Oil is less dense than
water thus it will be form at top layer after the centrifugation.
If
the oil phase is at high level , thus the separation phase will be shorter and
result in lower phase separation value.
Throughout
this experiment , there were some errors that occur. So , there are some
precaution step that we need to take as example we must make sure that the room
temperature of the laboratory is constant as it will affect the changing
properties of surfactants. Besides , we need to be aware of the parallax error
that might occur .
Since
the experiment of different volume of mineral use were done by different group
in the laboratory thus the result obtained might differ a bit than the
theoretical result.
Conclusion
1) Combination
of surfactants form a more stable emulsion than a single surfactant. Different
oily phase need a different value of HLB surfactant so that the most stable
emulsion can be formed.
2) Homogenization process
is applied in this mineral oil emulsion to reduce the size of droplets in
liquid-liquid dispersions. Before homogenization, the texture of emulsion is
non-homogenous, less consistency, more greasy in degree of oily
appearance and less spreading of colour. After homogenization, the texture of
emulsion is homogenous, more consistent, non greasy in degree of oily
appearance and more evenly spreading.
3) In oil-in-water emulsion, decrease in
temperature may cause high viscosity of an emulsion and vice versa. High
viscosity will increase the time for the separation of emulsion into two
layers.
4) As more volume of mineral oil is used in
formulation, a shorter separation phase will exhibit and this indicates a lower
phase separation value.
5) The higher the amount of mineral oil used,
the higher the amount of emulsifying agent (acacia) needed to lower the surface
interfacial tension.
6) The emulsion stability increases as the HLB
value increase because the droplet size decreases.
jReferences
1. http://chemistscorner.com/hlb-the-easiest-way-to-create-an-emulsion/
2. http://journal.scconline.org/pdf/cc1968/cc019n10/p00683-p00697.pdf
3. http://www.particlesciences.com/news/technical-briefs/2011/emulsion-stability-and-testing.html
4. Florence A.T. 1998.
Physicochemical Principle of Pharmacy, 3rd Edition
5. Aulton, M.E.
Pharmaceutics: The science of dosage form design. Edinburgh: Churchill
Livingstone.
6. http://medical-dictionary.thefreedictionary.com/Sudan+stain+test













No comments:
Post a Comment